Eradication of Prostate Cancer Using GenEpic™ (F-989)
NCT01987999
1st IRB – Prostate Study Results
GenEpic™ taken for 12 months
- 60 Prostate Cancer Patients, each confirmed with biopsy
- Each Patient took GenEpic™ daily for 12 months – No surgery, chemo or radiation
- Includes Patient Reports on
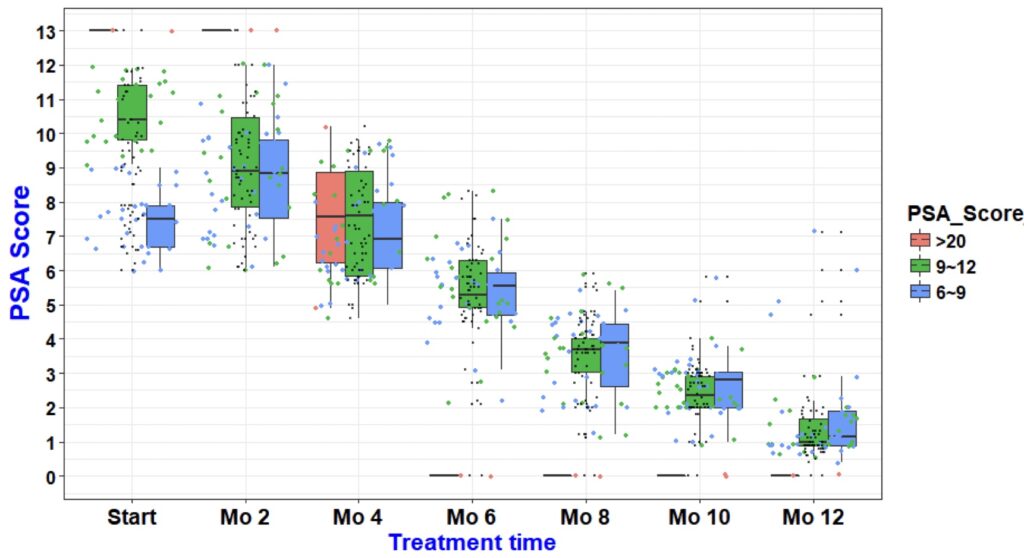
- PSA Scores
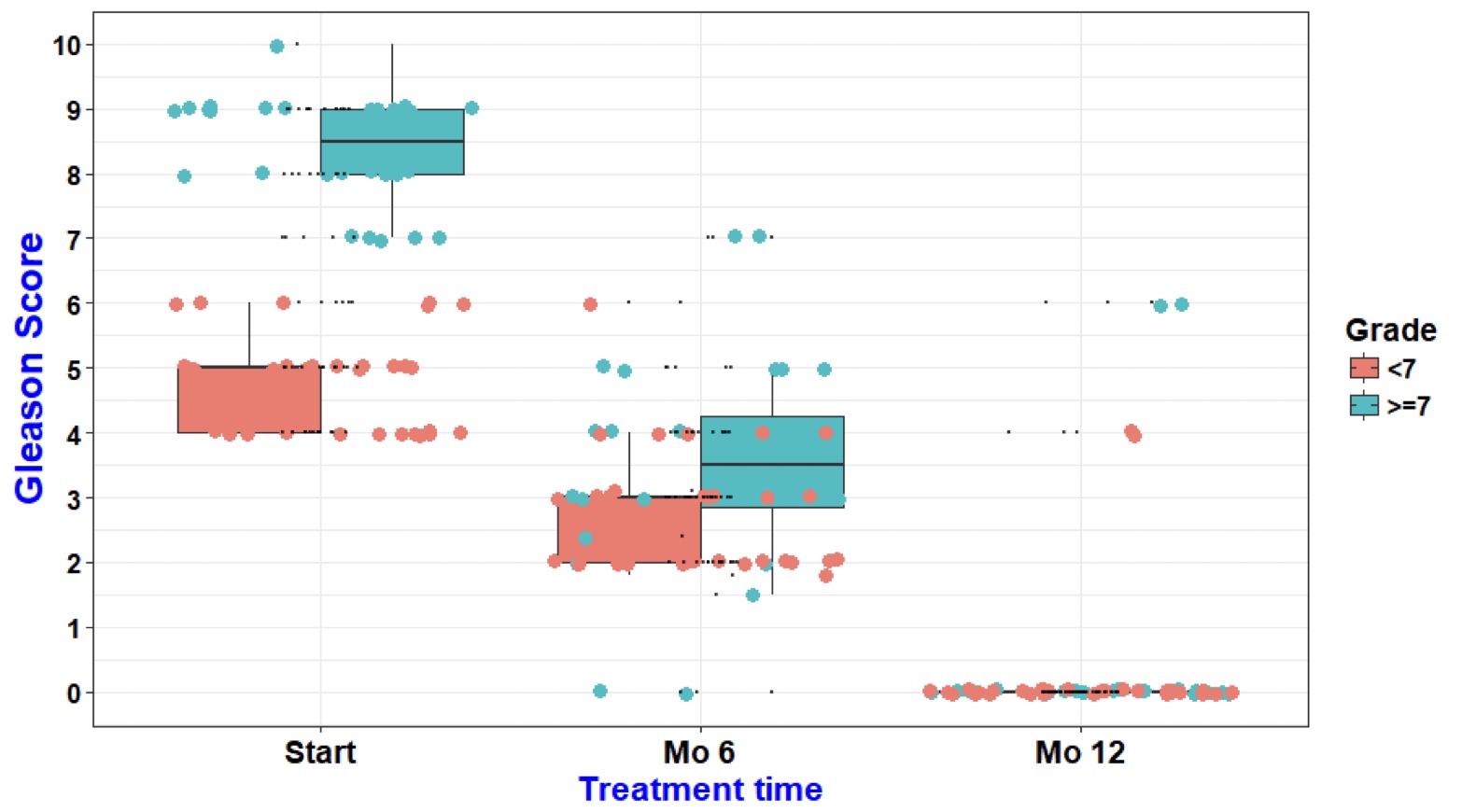
- Gleason Scores
- Negative Side Effects
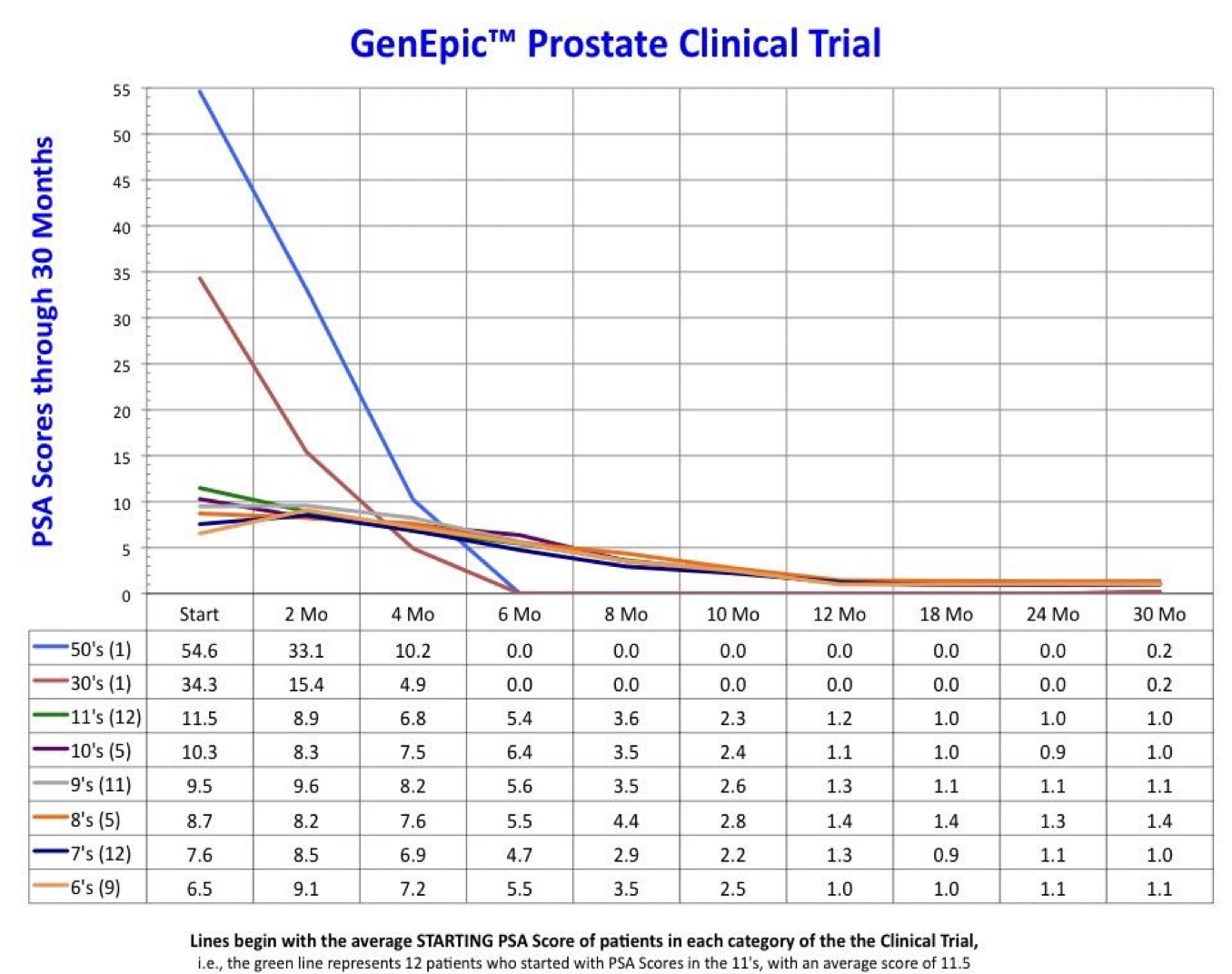
- Average PSA Scores dropped from 10.07 to 1.48
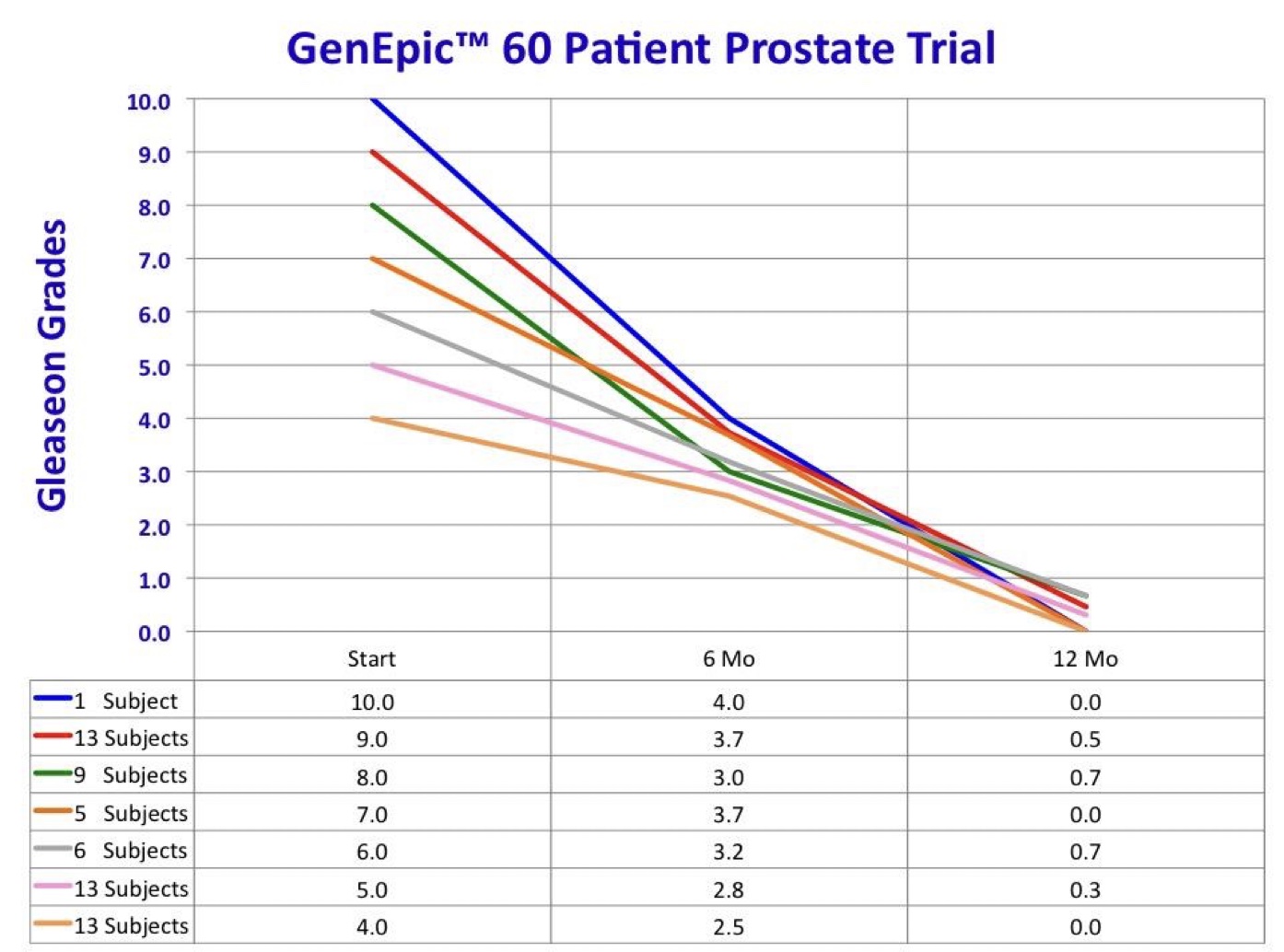
- Average Gleason Scores dropped from 6.45 to 0.33
- Example: A patient with an initial PSA of 54.6 and a Gleason Score of 10 now has a zero score in both categories.
- The 60 patients reported virtually no major negative side-effects