Eradication of Malignant Carcinoma in the Breast Tissue
NCT02286778
IRB – Double Blind Placebo Controlled Breast Cancer Clinical Trial
60 were enrolled, with an initial diagnoses of Stage I to Stage IV, Malignant Carcinoma in the Breast Tissue.
Each was confirmed by a Fine Needle Aspiration Biopsy (FNAB) or Breast Biopsy (BB), and MRI.
Participants were randomly assigned to one of three groups.
1. GenEpic™ Group – 1 packet of GenEpic™ twice daily, and dietary changes
2. Placebo Group – Took Placebo
3. Control Group – Took Nothing
No patient received surgery, chemo or radiation before or during the Clinical Trial.
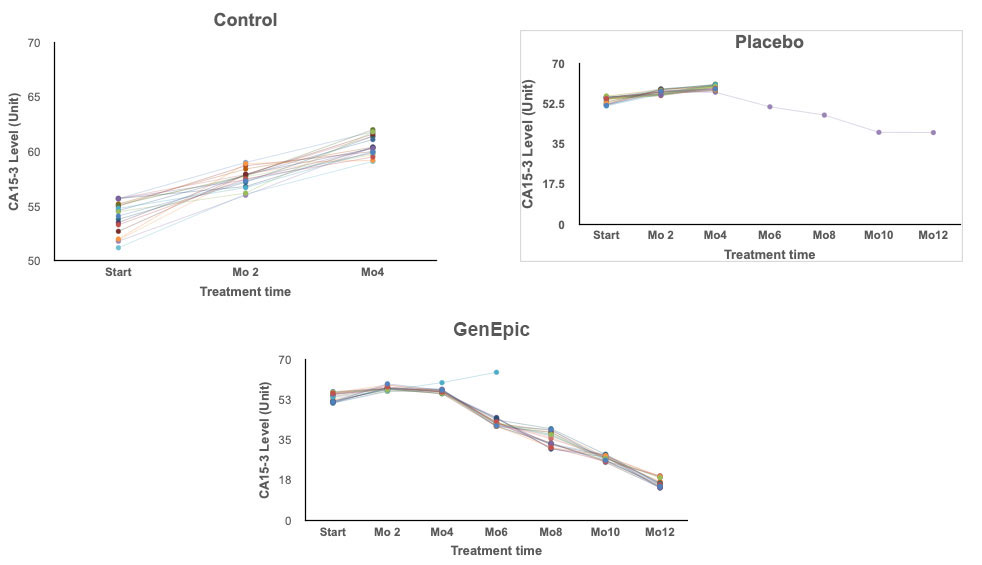
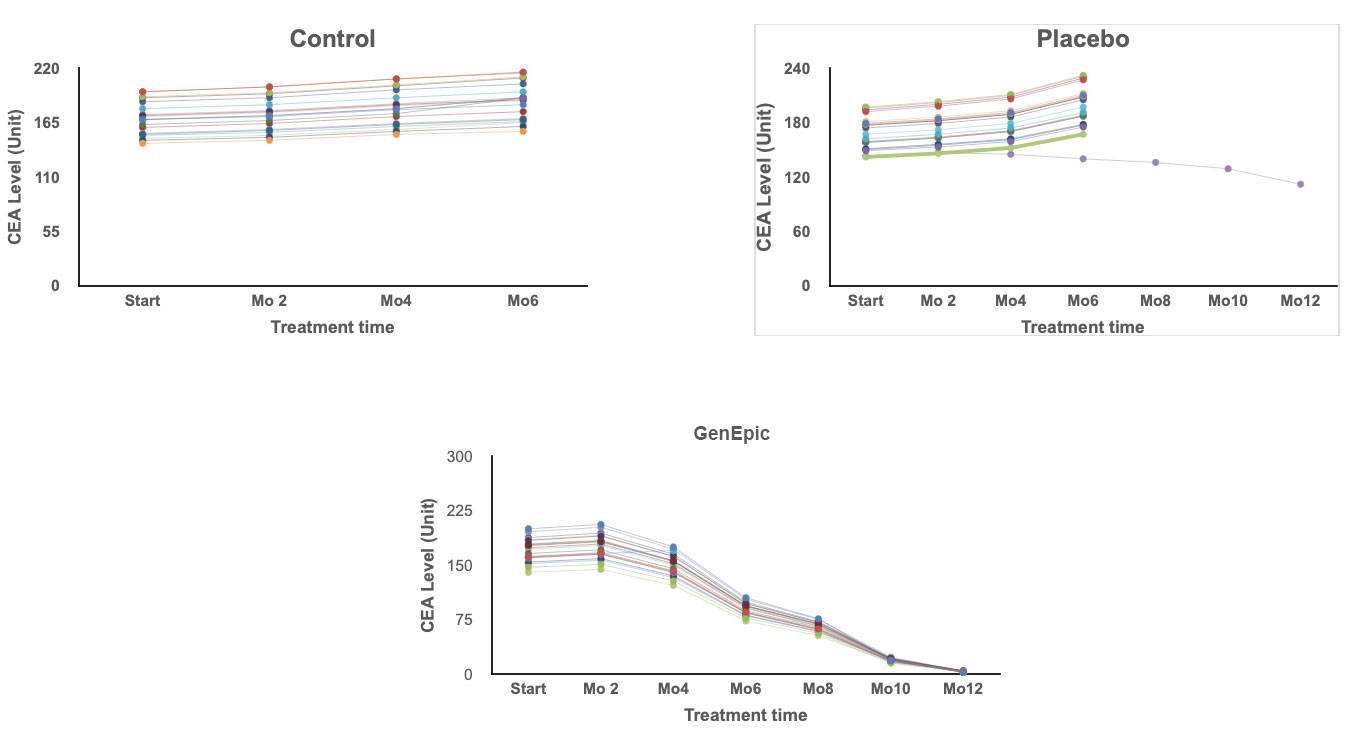
Group 1 - GenEpic™ Group
19 of the 20 patients experienced positive results. Decreased tumor size months 3 to 9. No discernible tumor at the end of 12 months.
Group 2 - Placebo Group
1 patient experienced tumor shrinkage during the 12 month period, 5 showed signs of no change. 15 experienced tumor enlargement. At 6 months the 15 unresponsive patients removed from of the clinical trial.
Group 3 - Control Group
No Treatment. No positive signs all participants removed from Study by the end of month 4.